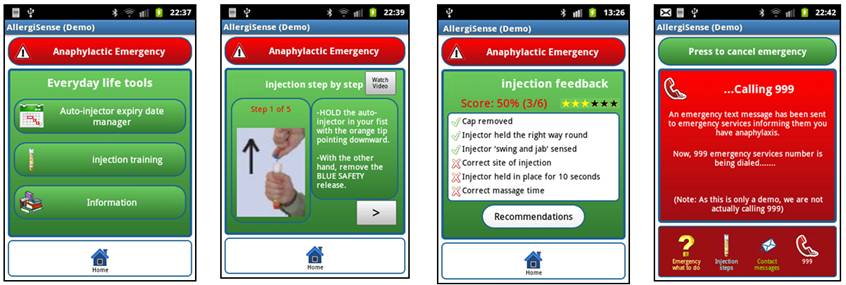
AllergiSense screenshots: (From left to right) Everyday life tools; Injection step-by-step instructions; Injection feedback from training injections using an AAI trainer device with Bluetooth(TM) sensors; Emergency tools.
Electronic, Electrical and Systems Engineering, University of Birmingham
Luis Hernandez-Munoz (Ph. D. researcher)1, Dr Sandra I. Woolley (Supervisor)1 and Dr Lavanya Diwakar (Consultant Immunologist, Specialist Advisor)2
1 Electronic, Electrical and Systems Engineering, University of Birmingham, UK.
2 Health Economics Unit, School of Health and Population Sciences, University of Birmingham, UK.
Emails: luisherdezm@yahoo.com.mx; s.i.woolley@bham.ac.uk; l.diwakar@bham.ac.uk
Anaphylaxis is a severe life-threatening allergic condition which is increasing in prevalence. The management of anaphylaxis requires the avoidance of allergen triggers and preparation in readiness for an anaphylactic reaction. People with anaphylaxis and their caregivers carry adrenaline auto-injectors ready for immediate administration in the event of an anaphylactic reaction. But, unfortunately, many people do not know how to use the auto-injectors and fail to use them or fail to use them correctly. This is due in part to deficiencies in training and also due to the lack of a system encouraging continuous practice and providing feedback on that practice. Pervasive healthcare technologies such as smartphone apps have demonstrated potential in the management of chronic conditions such as diabetes and cardiovascular disease. However, research into assistive technologies for the support of anaphylaxis management has been neglected.
AllergiSense is a system of smartphone tools that supports anaphylaxis management. It has been designed with the participation of clinicians, and people with anaphylaxis and their carers, and it has been evaluated quantitatively in terms of usability, performance and self-efficacy and qualitatively by clinical staff in five hospitals in the Midlands, UK. Its evaluation has provided evidence of the potential of smartphone tools to significantly improve adrenaline injection training skills and positively influence self-efficacy. In addition, the results provided insights into possible self-efficacy failings in traditional training and the benefits of embedding self-efficacy theory into the design process.
Ethical approval: Science, Technology, Engineering and Mathematics Ethical Review Committee of the University of Birmingham UK (ERN_13-1496).
Funding: The Anaphylaxis Campaign's Small Grant Scheme (04-13-LHM).
Sponsor: The University of Birmingham (RG_14-090).
Participating hospitals: The host hospital Leicester Royal Infirmary and Worcester Royal Hospital, University Hospitals Birmingham, Sandwell and West Birmingham Hospitals and Heart of England NHS Foundation Trust, Heartlands Hospital.
AllergySense clinical study was supported by the UK Clinical Research Network Study Portfolio (UKCRN)
http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=16894

AllergiSense screenshots: (From left to right) Everyday life tools; Injection step-by-step instructions; Injection feedback from training injections using an AAI trainer device with Bluetooth(TM) sensors; Emergency tools.
The importance of auto-injector (AAI) training is well reported in the literature [1]-[5]. However, in practice, training can be delayed or incomplete [6], or there may be an inadequate system of continuous practice [7]. Such problems with training can result in a lack of preparedness and failure in the use of auto-injectors [8]. Examples include a delay in administering adrenaline or using the injector incorrectly, carrying out unintentional injections (for instance, caused by holding the injector the wrong way up and injecting the holder's thumb), failing to hold the injector in place for the required time and failing to immediately call emergency services [9],[10]. The recommendation is that patients be properly trained and encouraged to regularly review injection instructions using instructional videos, and that family, carers and school staff receive the same training [7]. Mobile phone applications offer an opportunity to improve adrenaline injection training. Information-giving apps [11]-[16] have demonstrated a use for smartphone tools for anaphylaxis. However, there are no apps providing feedback on injection performance and encouraging maintenance of skills.
AllergiSense was designed with clinical and patient participation. It is a smartphone system for anaphylaxis management with adrenaline injection training. AllergiSense uses an auto-injector trainer fitted with wireless Bluetooth(TM) sensors that communicate with a smartphone to help people practice injections; reminding them to practice and providing feedback on each injection. The sensors can detect the removal of the injector cap and the motion of the injector trainer, for example, sensing the injection swing motion and sensing the trainer is held in place for ten seconds.
The AllergiSense app contains tools for the management of anaphylaxis in everyday life and emergency scenarios. For everyday life, the tools include a list of AAI expiry dates with reminders, videos about anaphylaxis and information about symptoms, a step-by-step trainer tool showing how to use an EpiPen(TM) AAI, and a trainer tool that provides feedback about practised injection steps from Bluetooth(TM) sensors mounted on the AAI trainer device. For emergency scenarios, an emergency 'what to do' list is included together with step-by-step instructions for AAI use, text messages (including the user's GPS location) can be sent to emergency contacts, and emergency services can be contacted with the touch of a '999' button.
Practice injections are checked by the smartphone application using the received wireless sensor data. AllergiSense does this using a data mining method that compares practice injections to correct injections provided by experts.
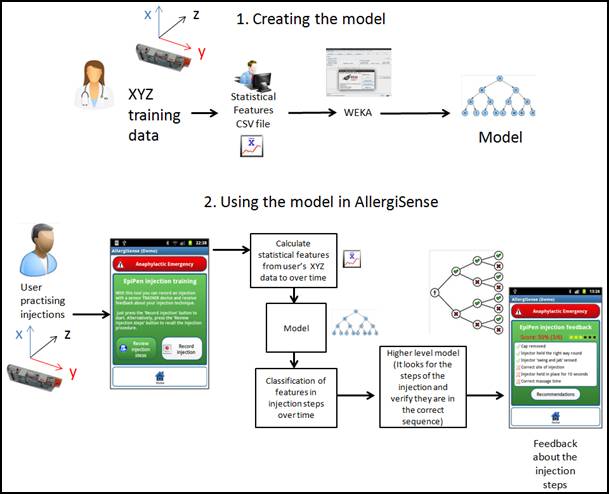
Modelling practice adrenaline injections and providing feedback on performance.
AllergiSense was implemented in an Android smartphone and the sensing unit uses an AAI trainer device with Bluetooth(TM)) accelerometer and pressure sensors and an Arduino "Pro mini" microcontroller.
The evaluation of AllergiSense involved two studies sponsored by the Anaphylaxis Campaign UK: a quantitative study with sixty-three healthy participants and a qualitative study with eight allergy specialists from five hospitals in the Midlands, UK. The main findings were:
Hernandez-Munoz, L.U. "Smartphone tools for anaphylaxis management". The University of Birmingham, 2014. Ph. D. thesis.
Hernandez-Munoz, L.U., Woolley, S.I. and Diwakar, L. "Pilot evaluation of Smartphone technology for adrenaline injection training". The British Society for Allergy and Clinical Immunology -BSCAI- Annual Meeting, 28th - 30th September, 2014. Telford UK.
Hernandez-Munoz, L.U. & Woolley, S.I. "Mobile phone tools with Ambient Intelligence for the management of life-threatening allergies". Book chapter on Human Aspects in Ambient Intelligence. Atlantis Press - Springer.
Hernandez-Munoz, L.U., Woolley, S.I. and Baber, C. "PervaLaxis Two: Encouraging anaphylactic people manage their own healthcare with a touchscreen personal mobile system". 5th International Conference on Ubiquitous Computing and Ambient Intelligence, UCAmI 2011. Riviera Maya, Mexico.
Hernandez Munoz, L.U., & Woolley, S.I. "A personal handheld device to support people with life-threatening anaphylactic allergies (PervaLaxis)". International Journal of Handheld Computing Research. Vol. 1. No. 1. IGI Publisher. January-March 2010.
Hernandez-Munoz, L.U. & Woolley, S.I. "A user-centred mobile health device to manage life-threatening anaphylactic allergies and provide support in allergic reactions". 9th International Conference on Information Technology and Applications in Biomedicine. ITAB'09. Cyprus.
Hernandez-Munoz, L.U., Woolley, S.I. & Baber, C. "A Mobile Health Device to Help People with Severe Allergies". Pervasive Health 2008. Ambient Technologies for Diagnosing and Monitoring Chronic Patients Workshop. Tampere, Finland.
Hernandez-Munoz, L.U., Woolley, S.I. & Baber, C. "A Mobile Context-Aware Device to Help People with Anaphylaxis". Second European Conference EuroSSC, Kendal, UK. Adjunct Proceedings. pp 24-25.
We sincerely thank the following for their support of the AllergiSense research, design and evaluation: The University of Birmingham, UK. The Mexican Council of Science and Technology (CONACyT), The Anaphylaxis Campaign UK (Small grant scheme), the Mexican Secretariat of Public Education (SEP), the Cadbury Schweppes Foundation, our clinical collaborators and our focus group and experimental participants.




